Targeting Matrix Metalloproteinases Using Tissue Inhibitors
TIMP-MMP contact surface
TIMP-MMP contact surface
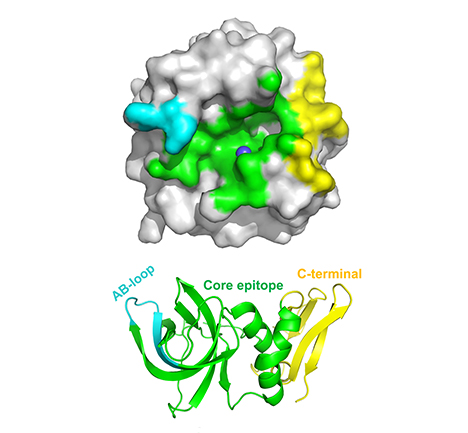
TIMP-MMP contact surface
TIMPs inhibit MMPs using a broad contact interface, as illustrated by TIMP-1 inhibition of MMP-10. The binding surface of TIMP-1 (bottom) involves three distinct protein regions: the core epitope (green), AB-loop (cyan) and C-terminal domain (yellow). The contact footprint of these binding epitopes on the catalytic domain of MMP-10 is shown in corresponding colors (top).
Despite proven and important roles for matrix metalloproteinases (MMPs) in promoting cancer progression, small-molecule MMP inhibitors have fared poorly in clinical trials. This is in part because of a lack of selectivity. This selectivity problem might be overcome by inhibitors highly selective for individual tumor-promoting MMP.
An alternative to small-molecule MMP inhibitor development could be the engineering of naturally occurring protein inhibitors of MMP. These are called tissue inhibitors of metalloproteinases (TIMPs). TIMPs offer an unparalleled scaffold for developing new MMP inhibitors of novel selectivity.
Unlike synthetic MMP inhibitors, tissue inhibitors of metalloproteinases feature a broad and extensive contact surface for interaction with MMP targets, encompassing more than 20 amino acid residues. This large interface offers an outstanding opportunity for molecular optimization.
Our lab collaborates with several other labs using both structure-guided approaches and directed evolution techniques. We're exploring the idea that TIMPs, like antibodies, can be engineered as targeted protein therapeutics of remarkable affinity and exquisite selectivity.
Our research team is working to develop selective inhibitors for several MMPs known to promote cancer progression by modifying the natural human TIMPs.
These targets include:
- MMP-3, a key inducer of epithelial-mesenchymal transition (EMT) and tumor progression in lung cancer and breast cancer.
- MMP-10, an enzyme necessary for lung tumors for maintenance of the cancer stem cell phenotype.
- MMP-9, a critical inducer of EMT, invasion, metastasis and angiogenesis in breast cancer and many other tumor types.